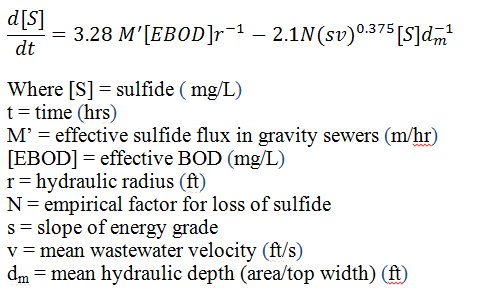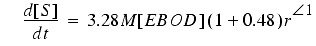Hydrogen-Sulfide (H2S) Modeling
Hydrogen sulfide forms in wastewater collection systems when anoxic conditions exist and results in corrosion, odor and toxicity problems. The rate of formation depends primarily on the strength of the wastewater as characterized by BOD concentration (Biochemical Oxygen Demand) and rate constants for the reactions which are dependent on temperature. Hydrogen sulfide in most commonly a problem in systems with long detention times in warm weather. The hydrogen sulfide calculations assume that sulfate concentration is not limiting.
The calculations are based on the Pomeroy-Parkhurst equation for predicting H2S concentrations in a sewer network; they can be used to evaluate the build-up or decay of the H2S concentration in a sewer system. The sewer system can be gravity and pressure combined system consisting of conduits, ponds, channels, wet-wells, pumps, pressure pipes and junctions.
The application procedures of Pomeroy equation for sewer system were well documented in the following publications:
- ASCE Manuals and Reports on Engineering Practice No. 69, Sulfide in Wastewater Collection and Treatment Systems
- EPA Design Manual EPA/625/I-85, Odor and Corrosion Control in Sanitary Sewerage System and Treatment Plants
The usual equation to predict sulfide formation is the Pomeroy-Parkhurst equation given in English units as:
The first term in the equation covers creation of sulfide while the second refers to loss. EBOD is essentially a temperature corrected BOD given by a modified van't Hoff-Arrhenius equation:
EBOD=BOD1.07T-20
Where:
In the case of surcharged pipes or force mains, the sulfide loss term goes away and the recommended equation is:
Where:
Values for coefficient such as M, M' and N depend on velocity, pH, dissolved oxygen concentration, amount of sediment in pipe, temperature and presence of other chemicals that can catalyze or inhibit the reaction, none of which are explicitly considered in the equations.
Approximate values of:
but values will vary widely. Values of these empirical coefficients should be calibrated for each model.
At node elements, the conventional mass balance equation is used:

Where:
The above mixing/mass-balacing equation is used to determine a node's outlet concentration for both H2S and BOD, for the storage type nodes (wet-well and pond), a H2S reaction process is also considered. Using the H2S determined from above mixing equation as inflow, S(in), a reaction equation is applied to determine an updated outflow H2S concentration, the reaction equation is:
S(out) = S(in) + dS/dt (dt)
In which:
dS/dt = k C(BOD) 1.07(T-20)
T is the temperature (Celsius), k is a user-specified reaction rate (1/hour), and dt is the detention time of the storage node.
Calculation assumptions: The Pomeroy equation was developed on steady flow condition. Since most models using SWMM solver are of dynamic modeling, some assumptions must be made for proper application of the Pomeroy equation. The primary assumption is that the solver will use the average hydraulic condition for the H2S calculations. The solver determines the average pipe flow by the total flow volume that has passed through the pipe for the simulation duration, the node H2S/BOD mixing calculations will also be based on the average flows of incoming and outlet pipes. The pipe detention time is determined by the pipe length and average flow velocity; the node detention time is determined by the average node volume and average outlet flow.
As a result of these assumptions the model provides a steady result set for the network which represents the expected average changes.
For details on running a H2S model, see H2S Modeling Workflow.