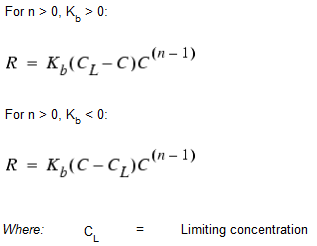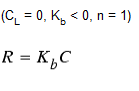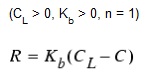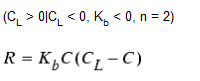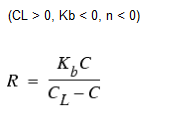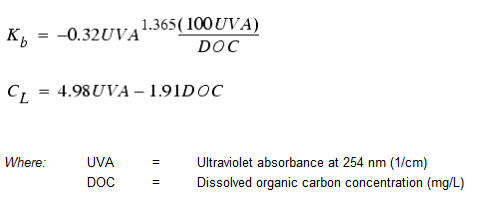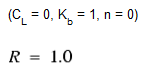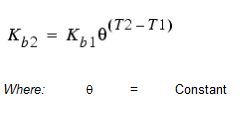Bulk Flow Reactions
While a substance moves down a pipe or resides in storage, it can undergo reaction with constituents in the water column. The rate of reaction can generally be described as a power function of concentration:
When a limiting concentration exists on the ultimate growth or loss of a substance, the rate expression becomes:
Some examples of different reaction rate expressions are:
Simple 1st-Order Decay
The decay of many substances, such as chlorine, can be modeled adequately as a simple first-order reaction.
First-Order Saturation Growth
This model can be applied to the growth of disinfection by-products, such as trihalomethanes, where the ultimate formation of by-product (CL) is limited by the amount of reactive precursor present.
Two-Component, 2nd-Order Decay
This model assumes that substance A reacts with substance B in some unknown ratio to produce a product P. The rate of disappearance of A is proportional to the product of A and B remaining. CL can be either positive or negative, depending on whether either component A or B is in excess, respectively. Clark (1998) has had success in applying this model to chlorine decay data that did not conform to the simple first-order model.
Michaelis-Menton Decay Kinetics
As a special case, when a negative reaction order n is specified, WaterGEMS will utilize the Michaelis-Menton rate equation, shown above for a decay reaction. (For growth reactions the denominator becomes CL + C.) This rate equation is often used to describe enzyme-catalyzed reactions and microbial growth. It produces first-order behavior at low concentrations and zero-order behavior at higher concentrations. Note that for decay reactions, CL must be set higher than the initial concentration present.
Koechling (1998) has applied Michaelis-Menton kinetics to model chlorine decay in a number of different waters and found that both Kb and CL could be related to the water's organic content and its ultraviolet absorbance as follows:
Zero-Order Growth
This special case can be used to model water age, where with each unit of time the concentration (i.e., age) increases by one unit.
The relationship between the bulk rate constant seen at one temperature (T1) to that at another temperature (T2) is often expressed using a van't Hoff-Arrehnius equation of the form:
In one investigation for chlorine, q was estimated to be 1.1 when T1 was 20 deg. C (Koechling, 1998).